Each year, the effects of corrosion on public and private assets represents an equivalent cost of 3.4% of global GDP, according to estimates.1 This staggering cost affects the infrastructure, energy, manufacturing, transportation, construction, marine, and service industries, costing manufacturers, operating companies, service providers, contractors, investors, engineers, designers, customers, taxpayers, and others involved in local, national, and global economies.
Corrosion engineers are engaged in the study and practice of corrosion management, providing guidance on the selection of cost-effective materials, coatings, and corrosion mitigation strategies. The work performed by these specialists helps to ensure the technical integrity of materials and installations. In determining appropriate materials, coatings, and corrosion management tactics, the specialist must assess each component of a system against a variety of factors, including environmental conditions, application requirements, estimated product/system lifetime, and available mitigation methods.
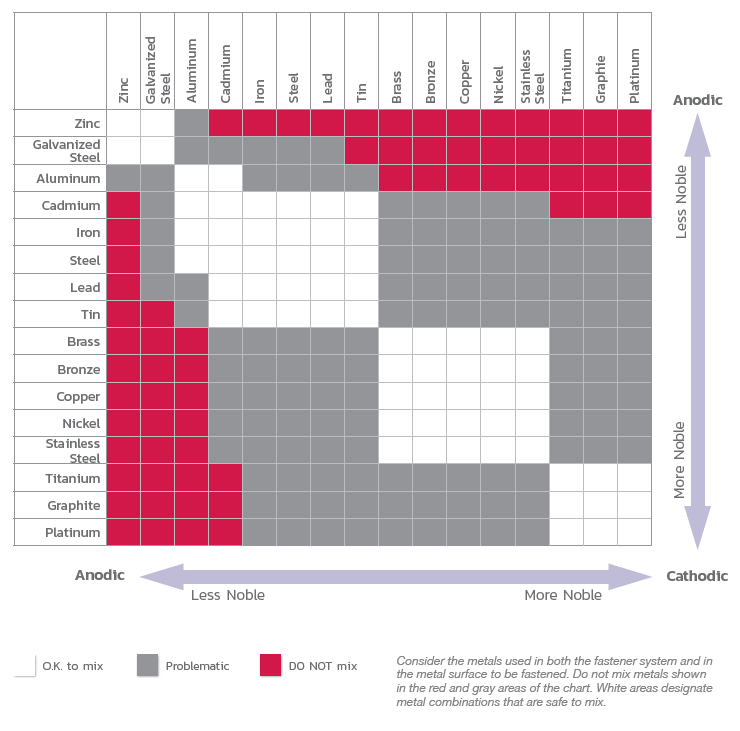
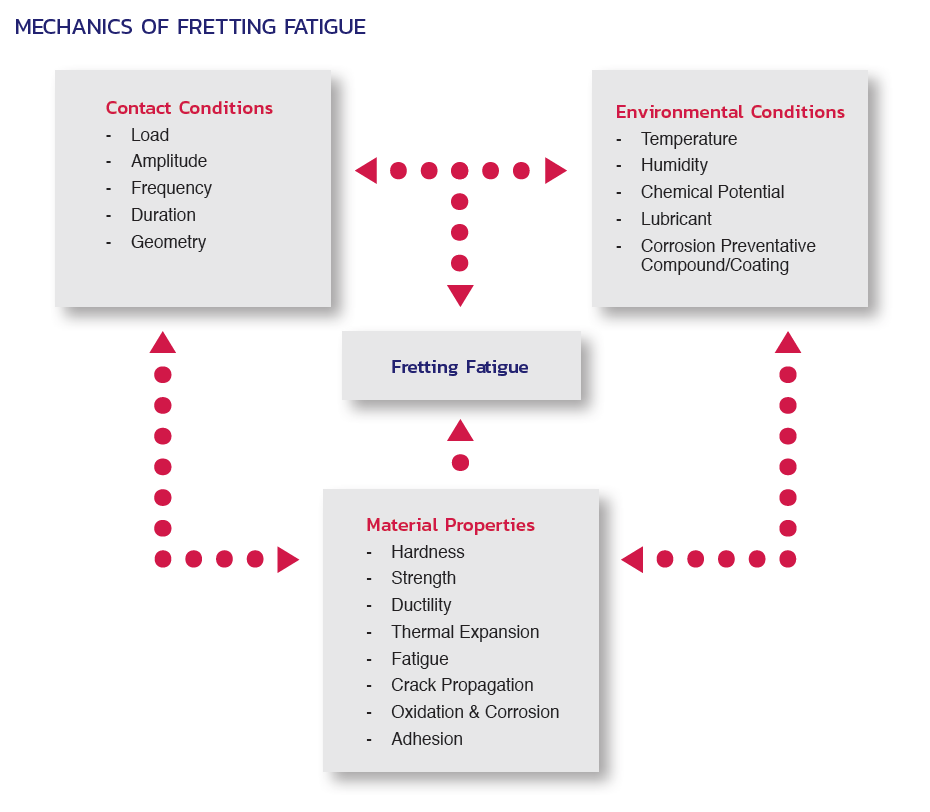
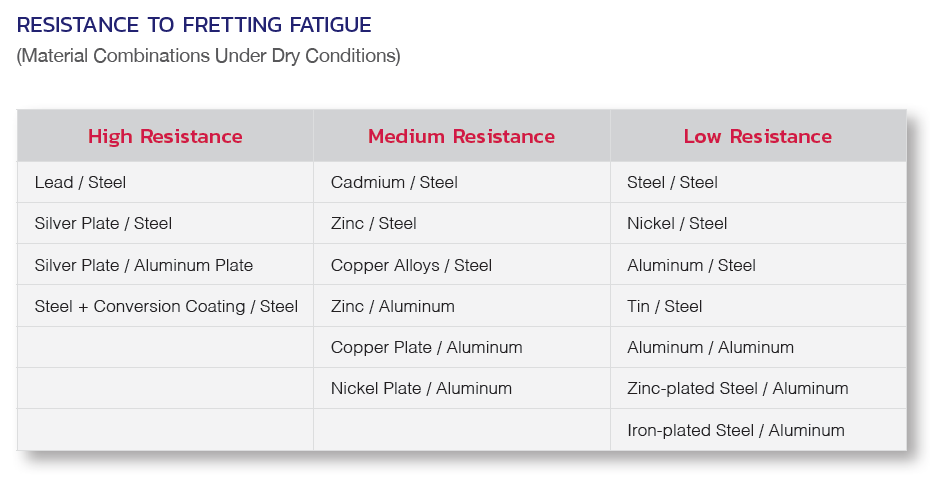
Fasteners – often the least expensive component in a system’s design – deserve particular attention in that (a) their failure can lead to serious expense and performance issues affecting the entire installation, and (b) this failure can largely be avoided or mitigated through the proper selection of materials, coatings, and platings, to help ensure desired performance over the life of the installation.
This document will discuss some of the important considerations involved in the selection of fastener materials, designs, and coatings, in order to help design engineers, project managers, and purchasing personnel avoid potential problems that can result from fastener corrosion.
1 National Association of Corrosion Engineers (NACE) NACE International has become the global leader in developing corrosion prevention and control standards, certification and education. NACE is a standards-writing organization accredited by the American National Standards Institute (ANSI).

 Atmospheric Environments
Atmospheric Environments Water Environments
Water Environments Soil Environments
Soil Environments Uniform corrosion (also referred to as “general” corrosion and “general attack” corrosion) tends to develop uniformly over an exposed surface, resulting in the thinning of materials, until failure occurs. Uniform corrosion is dependent upon two factors: the composition of the material and the characteristics of the environment.
Uniform corrosion (also referred to as “general” corrosion and “general attack” corrosion) tends to develop uniformly over an exposed surface, resulting in the thinning of materials, until failure occurs. Uniform corrosion is dependent upon two factors: the composition of the material and the characteristics of the environment.